Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors.
http://www.nejm.org/doi/full/10.1056/NEJMoa1607427
Strosberg J1, El-Haddad G1, Wolin E1, Hendifar A1, Yao J1, Chasen B1, Mittra E1, Kunz PL1, Kulke MH1, Jacene H1, Bushnell D1, O'Dorisio TM1, Baum RP1, Kulkarni HR1, Caplin M1, Lebtahi R1, Hobday T1, Delpassand E1, Van Cutsem E1, Benson A1, Srirajaskanthan R1, Pavel M1, Mora J1, Berlin J1, Grande E1, Reed N1, Seregni E1, Öberg K1, Lopera Sierra M1, Santoro P1, Thevenet T1, Erion JL1, Ruszniewski P1, Kwekkeboom D1, Krenning E1; NETTER-1 Trial Investigators.
Abstract
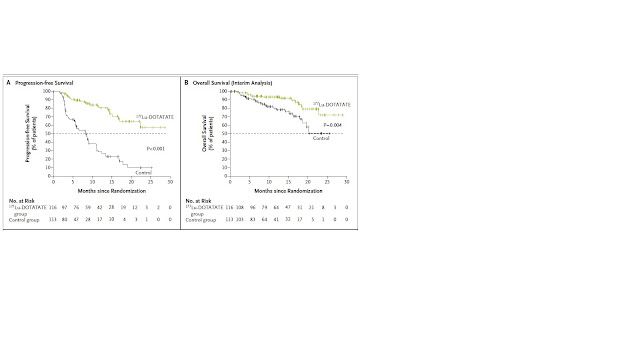
Background Patients with advanced midgut neuroendocrine tumors who have had disease progression during first-line somatostatin analogue therapy have limited therapeutic options. This randomized, controlled trial evaluated the efficacy and safety of lutetium-177 (177Lu)-Dotatate in patients with advanced, progressive, somatostatin-receptor-positive midgut neuroendocrine tumors. Methods We randomly assigned 229 patients who had well-differentiated, metastatic midgut neuroendocrine tumors to receive either 177Lu-Dotatate (116 patients) at a dose of 7.4 GBq every 8 weeks (four intravenous infusions, plus best supportive care including octreotide long-acting repeatable [LAR] administered intramuscularly at a dose of 30 mg) (177Lu-Dotatate group) or octreotide LAR alone (113 patients) administered intramuscularly at a dose of 60 mg every 4 weeks (control group). The primary end point was progression-free survival. Secondary end points included the objective response rate, overall survival, safety, and the side-effect profile. The final analysis of overall survival will be conducted in the future as specified in the protocol; a prespecified interim analysis of overall survival was conducted and is reported here. Results At the data-cutoff date for the primary analysis, the estimated rate of progression-free survival at month 20 was 65.2% (95% confidence interval [CI], 50.0 to 76.8) in the 177Lu-Dotatate group and 10.8% (95% CI, 3.5 to 23.0) in the control group. The response rate was 18% in the 177Lu-Dotatate group versus 3% in the control group (P<0.001). In the planned interim analysis of overall survival, 14 deaths occurred in the 177Lu-Dotatate group and 26 in the control group (P=0.004). Grade 3 or 4 neutropenia, thrombocytopenia, and lymphopenia occurred in 1%, 2%, and 9%, respectively, of patients in the 177Lu-Dotatate group as compared with no patients in the control group, with no evidence of renal toxic effects during the observed time frame. Conclusions Treatment with 177Lu-Dotatate resulted in markedly longer progression-free survival and a significantly higher response rate than high-dose octreotide LAR among patients with advanced midgut neuroendocrine tumors. Preliminary evidence of an overall survival benefit was seen in an interim analysis; confirmation will be required in the planned final analysis. Clinically significant myelosuppression occurred in less than 10% of patients in the 177Lu-Dotatate group. (Funded by Advanced Accelerator Applications; NETTER-1 ClinicalTrials.gov number, NCT01578239 ; EudraCT number 2011-005049-11 .).




Comentários
Postar um comentário